Apr 2024 – Nov 2025
Master’s thesis
on the topic:
“Development of a multimodal system based on optical coherence tomography and autofluorescence imaging”
Annegret Umann
An image can reveal where a lesion lies; its chemistry hints at why. Diagnostic imaging advances fastest when anatomy and biochemistry are captured together – quickly, gently, and in register.
This thesis developed a benchtop multimodal microscope that integrated optical coherence tomography (OCT), spectrally resolved autofluorescence (AF) imaging, and co-registered brightfield. The rationale was direct: OCT provided label-free, depth-resolved morphology with micrometer-scale resolution and low photodose, while AF reported endogenous biochemical and metabolic signatures by exciting native fluorophores and recording their emission spectra. Considered alone, each modality leaves gaps; combined in a single optical path, they yield aligned 3D structure and spatially resolved spectra that can better differentiate tissue states.
The thesis was driven by three concrete aims: (i) it specified system requirements and wavelength ranges, then realized a robust OCT–AF instrument using a laser-scanning microscope as the sample arm; (ii) it implemented synchronized acquisition and processing pipelines for OCT volumes and AF spectra/images; and (iii) it quantitatively characterized resolution, field of view, sensitivity, volume rate, and photodose and demonstrated capability on biological specimens. The benchtop architecture emphasized a comparatively large field of view and rapid prototyping, complementing catheter and handheld-based multimodal systems reported in the literature. An additional objective was to provide a platform for preparatory and comparative experiments within OptoCarDi, a project pursuing a multimodal catheter for minimally invasive assessment of myocarditis.
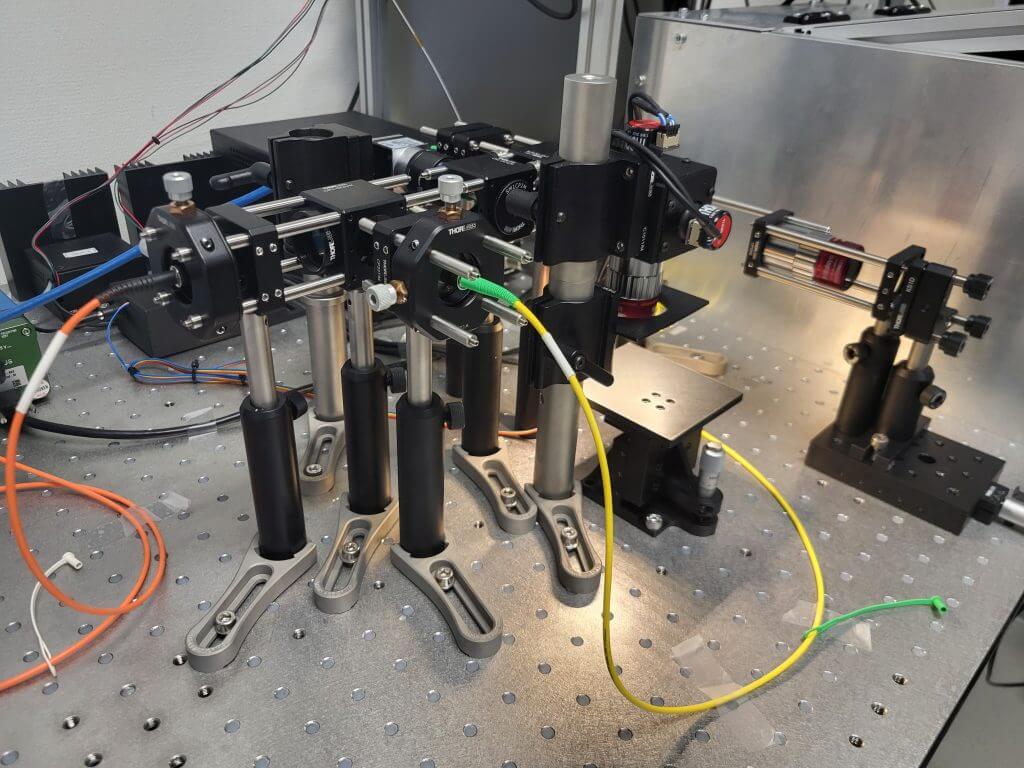
By fusing depth-resolved morphometry with endogenous spectral contrast, the system was intended to improve the discrimination of pathological vs. healthy tissue, e.g., early inflammatory or neoplastic change, while remaining non-contact and tissue-sparing. Beyond immediate use cases, the open, modular design invited adaptation to diverse samples and future extensions (such as lifetime or Raman channels), enabling cross-disciplinary studies that link what tissues look like to what cells are doing.

